|
 |
 |
 |
Taylor K-1690ChemWorld is a distributor of Taylor Technologies Test Kits and Reagents. Order directly online and save today.
DROP TEST
>SODIUM NITRITE (1 drop = 40 ppm)
COMPONENTS:
1 x 5011 Instruction
1 x 9198R Sample Tube, Graduated, 25 mL, plastic
w/cap and red dot
1 x R-0819-C Ferroin Indicator, 2 oz, DB
2 x R-0820-C CAN Solution, 2 oz, DB.
1. Rinse and fill sample tube (#9198R) to 5 mL
mark with cooled (room temperature) water to be
tested.
2. Add 4 drops R-0819 Ferroin Indicator. Swirl to
mix. Sample should turn red (orange).
3. Add R-0820 CAN Solution dropwise, swirling and
counting after each drop, until color changes from
red (orange) to blue. Always hold bottle in
vertical position.
4. Multiply drops of R-0820 CAN Solution by 40. Record
as parts per million (ppm) sodium nitrite.
NOTE: For results as nitrite, multiply sodium nitrite
concentration by 0.67.
DROP TEST
> PHOSPHONATE EQUIVALENCE (PPM)
COMPONENTS:
1 x 5051 Instruction
1 x 9198P Sample Tube, Graduated, 25 mL, plastic w/cap
and purple dot
1 x 9315 Test Paper, pH, 1.8-3.8
1 x R-0686P-C Sulfuric Acid N (purple cap), 2 oz, DB
1 x R-0697-C Thiosulfate N/10, 2 oz, DB
1 x R-0802P-I XO Indicator Powder, 10 g
1 x R-0803-C Phosphonate Titrating Solution, 2 oz, DB
1 x R-0805-C Fluoride Masking Agent, 2 oz, DB.
NOTE: Iron can cause negative interference at a level greater
than 5 ppm.
Orthophosphate and polyphosphate can cause
positive interference at all levels.
NOTE: Run a blank using tap water. Normal blank
requires about 2 drops of R-0803 Phosphonate Titrating
Solution to reach endpoint.
1. Rinse and fill 25 mL sample tube (#9198P) to 25 mL
mark with water to be tested.
2. Add:
1 drop R-0697 Thiosulfate N/10
10 drops R-0805 Fluoride Masking Agent
1 level dipper R-0802P XO Indicator Powder
Swirl to mix.
3. Adjust pH between 2.6 and 3.0:
Add 1 drop R-0686P Sulfuric Acid N. Swirl to mix.
Dip test paper (#9315) into sample, in direction of
arrow, for 3 seconds, with all color zones immersed.
Match indicator zone (unnumbered square between 2.7
and 3.0 color standards) with color scale. Read printed
pH value. If necessary, continue adding R-0686P Sulfuric
Acid N dropwise, swirling and checking pH with a new test
paper after each drop, until a pH between 2.6 and 3.0 is
obtained. Sample should be yellow.
4. Add R-0803 Phosphonate Titrating Solution dropwise,
swirling and counting after each drop, until color changes
from yellow to purple-pink. Always hold bottle in
vertical position.
5. Subtract drops of R-0803 Phosphonate Titrating Solution in
blank from drops in sample (Step 4). Multiply
by appropriate conversion factor (see CONVERSION FACTORS).
Record as parts per million (ppm) phosphonate.
DROP TEST
> TOTAL HARDNESS (1 drop = 10 ppm)
COMPONENTS:
1 x 5202 Instruction
1 x 9198B Sample Tube, Graduated, 25 mL, plastic w/cap
and blue dot
1 x R-0619B-C Hardness Buffer (blue cap), 2 oz, DB
1 x R-0620B-I Hardness Indicator Powder (blue cap), 10 g
1 x R-0683-C Hardness Reagent, 2 oz, DB.
1. Rinse and fill 25 mL sample tube (#9198B) to 25 mL
mark with water to be tested.
NOTE: For results in grains per gallon (gpg), fill to
14.6 mL mark.
2. Add 5 drops R-0619B Hardness Buffer. Swirl to mix.
3. Add 1 dipper R-0620B Hardness Indicator Powder.
Swirl until dissolved. If hardness is present,
sample will turn red.
4. Add R-0683 Hardness Reagent dropwise, swirling and
counting after each drop, until color changes from
red to blue. Always hold bottle in vertical
position.
5. Multiply drops of R-0683 Hardness Reagent by 10.
Record as parts per million (ppm) total hardness as
calcium carbonate.
NOTE: For 14.6 mL sample, record drops as grains per
gallon (gpg) total hardness as calcium carbonate.
DROP TEST SODIUM SULFITE (1 drop = 10 ppm)
COMPONENTS:
1 x 5203 Instruction
1 x 9198W Sample Tube, Graduated, 25 mL, plastic w/cap
and white dot
1 x R-0699-C Iodide Iodate Reagent, 2 oz, DB
1 x R-0638W-C Phenolphthalein Indicator (white cap),
2 oz, DB
1 x R-0725-I Acid Starch Indicator Powder, 10 g.
1. Collect water to be tested in a clean, preferably
large-mouthed, bottle to overflowing. Immediately
cap and cool to room temperature.
2. Rinse and fill 25 mL sample tube (#9198W) to 25 mL
mark with cooled (room temperature) water to be tested.
NOTE: For results in grains per gallon (gpg), fill to
14.6 mL mark.
3. Add 1 drop R-0638W Phenolphthalein Indicator. Swirl
to mix. Sample should turn red.
4. Add R-0725 Acid Starch Indicator Powder, a dipper at
a time, swirling after each dipper, until color
changes from red to colorless. Add 2 more dippers.
Swirl until dissolved.
5. Add R-0699 Iodide Iodate Reagent dropwise, swirling
and counting after each drop, until color changes
from colorless to a faint but permanent blue.
Always hold bottle in vertical position.
6. Multiply drops of R-0699 Iodide Iodate Reagent by
10. Record as parts per million (ppm) sodium
sulfite. For 14.6 mL sample, record drops as
grains per gallon (gpg) sodium sulfite.
DROP TEST
> CHLORIDE (1 drop = 10 ppm)
COMPONENTS:
1 x 5212C Instruction
1 x 9198O Sample Tube, Graduated, 25 mL, plastic
w/cap and orange dot
1 x R-0630-C Chromate Indicator, 2 oz, DB
1 x R-0638O-A Phenolphthalein Indicator (orange cap),
.75 oz, DB
1 x R-0706-C Silver Nitrate Reagent, 2 oz, DB
1 x R-0736O-C Sulfuric Acid .6N (orange cap), 2 oz, DB.
When sulfite content of sample water to be tested
exceeds 10 ppm, the sulfite should be oxidized to
prevent interference in test. A 25 mL sample is
first adjusted to the appropriate pH, then 1 mL
(or 24 drops) of R-0649 3% Hydrogen Peroxide
Solution (sold separately) is added and thoroughly
mixed. Continue with the rest of the procedure.
1. Rinse and fill 25 mL sample tube (#9198O) to 25 mL
mark with water to be tested.
NOTE: For results in grains per gallon (gpg), fill to
14.6 mL mark.
2. Add 2 drops R-0638O Phenolphthalein Indicator. If
colorless--proceed to Step 3. If red, add R-0736O
Sulfuric Acid .6N dropwise, swirling after
each drop, until color changes from red to
colorless.
3. Add 5 drops R-0630 Chromate Indicator. Swirl to
mix. Sample should turn yellow.
4. Add R-0706 Silver Nitrate Reagent dropwise,
swirling and counting after each drop, until color
changes from yellow to a milky salmon (brick) red.
Always hold bottle in vertical position.
NOTE: Do not add enough R-0706 Silver Nitrate Reagent
to give a brown color. First change from yellow
to a milky salmon (brick) red is the endpoint.
5. Multiply drops of R-0706 Silver Nitrate Reagent by
10. Record as parts per million (ppm) chloride.
For 14.6 mL sample, record drops as grains per
gallon (gpg) chloride.
DROP TEST
> P/T ALKALINITY (HCl) (1 drop = 10 ppm)
COMPONENTS:
1 x 5229C Instruction
1 x 9198G Sample Tube, Graduated, 25 mL, plastic w/cap
and green dot
1 x R-0638G-C Phenolphthalein Indicator (green cap),
2 oz, DB
1 x R-0645-C Total Alkalinity Indicator, 2 oz, DB
1 x R-0724-C Hydrochloric Acid .12N, 2 oz, DB.
1. Rinse and fill 25 mL sample tube (#9198G) to 25 mL
mark with water to be tested.
NOTE: For results in grains per gallon (gpg), fill to
14.6 mL mark.
2. Add 3 drops R-0638G Phenolphthalein Indicator.
Swirl to mix. Sample will turn pink if P
alkalinity is present--proceed to Step 3. If
colorless, go to Step 4.
3. If pink, add R-0724 Hydrochloric Acid .12N
dropwise, swirling and counting after each drop,
until color changes from pink to colorless. Record
drops as P reading. Always hold bottle in vertical
position.
4. Add 5 drops R-0645 Total Alkalinity Indicator.
Swirl to mix. Sample should turn green.
5. Add R-0724 Hydrochloric Acid .12N dropwise,
swirling and counting after each drop, until color
changes from green to red. Record total drops
(Steps 3 and 5) as T reading. Always hold bottle in
vertical position.
6. Multiply P reading by 10. Record as parts per
million (ppm) P alkalinity as calcium carbonate.
Multiply T reading by 10. Record as ppm T
alkalinity as calcium carbonate. For 14.6 mL
sample, record P reading as grains per gallon (gpg)
P alkalinity as calcium carbonate. Record T
reading as gpg T alkalinity as calcium carbonate.
MIDGET COMPARATOR TEST SILICA (5-50, 25-250 or 50-500 ppm)
MIDGET:
9257 (5, 10, 15, 20, 25, 30, 40, 50 ppm)
REAGENTS:
1 x R-1305Q-II Silica Reagent #4, 50 g
1 x R-1305U-C Silica Reagent #3*, 2 oz
1 x R-1306T-C Silica Reagent #1**, 2 oz
1 x R-1306U-C Silica Reagent #2, 2 oz
APPARATUS:
1 x 3267 Cap, Test Cell, 5 mL, plastic
1 x 4005 Cylinder, Graduated, 50 mL (1 mL div.), glass
1 x 4025 Test Cell, Calibrated 5 mL, plastic
1 x 4026 Dipper, plastic, large
3 x 4030 Pipets, Calibrated 0.5 & 1.0 mL plastic
w/cap
1 x 5321 Instruction
1 x 6002 Brush, Cell Cleaner
1 x 9198 Sample Tube, Graduated, 25 mL, plastic w/cap.
For 5-50 ppm Silica
1. Rinse and fill 25 mL sample tube (#9198) to 25 mL
mark with water to be tested.
2. Using a 1.0 mL pipet (#4030), add 1.0 mL R-1306T
Silica Reagent #1**. Swirl to mix.
3. Using a separate 1.0 mL pipet, add 1.0 mL R-1306U
Silica Reagent #2. Swirl to mix. WAIT 5 MINUTES.
4. Using a separate 1.0 mL pipet, add 1.0 mL R-1305U
Silica Reagent #3*. Swirl to mix.
5. Using a large dipper (#4026), add 1 level dipper
R-1305Q Silica Reagent #4. Swirl until dissolved.
WAIT 1 MINUTE. Transfer to 5 mL test cell (#4025)
to 5 mL mark.
6. Wipe dry and place in comparator WITH FROSTED SIDE
FACING OPERATOR.
7. Match color in test cell with a color standard.
Record as parts per million (ppm) silica.
For 5-250 ppm Silica
1. Rinse and fill 50 mL graduated cylinder (#4005) to
10 mL mark with water to be tested.
2. Dilute to 50 mL mark with distilled, deionized, or
silica-free water. Pour back and forth into 25 mL
sample tube (#9198) to mix. Transfer to 25 mL
sample tube to 25 mL mark.
3. Using a 1.0 mL pipet (#4030), add 1.0 mL R-1306T
Silica Reagent #1**. Swirl to mix.
4. Using a separate 1.0 mL pipet, add 1.0 mL R-1306U
Silica Reagent #2. Swirl to mix. WAIT 5 MINUTES.
5. Using a separate 1.0 mL pipet, add 1.0 mL R-1305U
Silica Reagent #3*. Swirl to mix.
6. Using a large dipper (#4026), add 1 level dipper
R-1305Q Silica Reagent #4. Swirl until dissolved.
WAIT 1 MINUTE. Transfer to 5 mL test cell (#4025)
to 5 mL mark.
7. Wipe dry and place in comparator WITH FROSTED SIDE
FACING OPERATOR.
8. Match color in test cell with a color standard.
Multiply reading by 5. Record as parts per million
(ppm) silica.
For 5-500 ppm Silica
1. Rinse and fill 50 mL graduated cylinder (#4005) to
5 mL mark with water to be tested.
2. Dilute to 50 mL mark with distilled, deionized, or
silica-free water. Pour back and forth into 25
mL sample tube (#9198) to mix. Transfer to 25 mL
tube to 25 mL mark.
3. Using a 1.0 mL pipet (#4030), add 1.0 mL R-1306T
Silica Reagent #1**. Swirl to mix.
4. Using a separate 1.0 mL pipet, add 1.0 mL R-1306U
Silica Reagent #2. Swirl to mix. WAIT 5 MINUTES.
5. Using a separate 1.0 mL pipet, add 1.0 mL R-1305U
Silica Reagent #3*. Swirl to mix.
6. Using a large dipper (#4026), add 1 level dipper
R-1305Q Silica Reagent #4. Swirl until dissolved.
WAIT 1 MINUTE. Transfer to 5 mL test cell (#4025)
to 5 mL mark.
7. Wipe dry and place in comparator WITH FROSTED SIDE
FACING OPERATOR.
8. Match color in test cell with a color standard.
Multiply reading by 10. Record as parts per
million (ppm) silica.
*WARNING: Silica Reagent #3 (R-1305U) contains 10%
oxalic acid, a poison.
**WARNING: Silica Reagent #1 (R-1306T) contains 14.7%
(w/v) hydrochloric acid, a corrosive acid.
DROP TEST
> MOLYBDENUM (1 drop = 2, 5, 20 or 50 ppm)
COMPONENTS:
1 x 4029 Pipet, Calibrated 0.5 & 1.0 mL, plastic
2 x 4030 Pipets, Calibrated 0.5 & 1.0 mL, plastic
w/cap
1 x 4078 Pipet, Graduated, 3 mL (0.5 mL div.),
plastic
1 x 5359 Instruction
3 x 9198 Sample Tubes, Graduated, 25 mL, plastic
w/cap
1 x R-0890 Molybdenum Buffer Solution
1 x R-0892 Molybdenum Titrating Solution, DB
1 x R-0900 Molybdenum Indicator Powder
1 x R-0901 Molybdenum Indicator Solvent.
Molybdenum Indicator Solution Preparation:
For 1 drop = 2, 20, or 50 ppm Mo
Using a 1.0 mL pipet (#4030), add 2.5 mL
R-0901 Molydenum
Indicator Solvent to a clean 25 mL sample tube. Add 5
level dippers R-0900 Molybdenum Indicator Powder. Swirl
until solution turns a clear, red-orange color.
Undissolved crystals will remain in the solvent-powder
mixture.
For 1 drop = 5 ppm Mo
Using a 1.0 mL pipet (#4030), add 1.5 mL
R-0901 Molydenum
Indicator Solvent to a clean 25 mL sample tube. Add 3
level dippers R-0900 Molybdenum Indicator Powder. Swirl
until solution turns a clear, red-orange color.
Undissovled crystals will remain in the solvent-powder
mixture.
For 1 drop = 2 ppm Mo
1. Rinse and fill a clean 25 mL sample tube (#9198) to
25 mL mark with distilled, deionized, or
molybdenum-free tap water. This will be the blank.
2. Rinse and fill a second clean 25 mL sample tube to
25 mL mark with water to be tested.
3. Using a 1.0 mL pipet (#4030), add 1.0 mL R-0890
Molybdenum Buffer Solution to each 25 mL sample
tube. Swirl to mix.
4. Using a separate 1.0 mL pipet (#4029), add 1.0 mL
Molybdenum Indicator Solution (prepared above) to
each sample tube, transferring as few undissolved
crystals as possible. However, a few crystals that
may be transferred will not affect results. Swirl
to mix. The blank should turn peach and the sample
will turn red-orange to red if molybdenum is present.
5. Add R-0892 Molybdenum Titrating Solution, dropwise,
swirling and counting after each drop, to sample
tube containing water sample, until sample color
matches blank color, or until no further change in
color occurs. Always hold bottle in vertical position.
6. Multiply drops of R-0892 Molybdenum Titrating
Solution by 2. Record as parts per million (ppm)
molybdenum.
NOTE: To convert molybdenum (Mo) readings to molybdate
(MoO4), multiply Mo readings by 1.7; to convert
to sodium molybdate dihydrate (Na2MoO4.2H2O),
multiply by 2.52.
For 1 drop = 5 ppm Mo
1. Rinse and fill a clean 25 mL sample tube (#9198) to
10 mL mark with distilled, deionized, or
molybdenum-free tap water. This will be the blank
2. Rinse and fill a second clean 25 mL sample tube to
10 mL mark with water to be tested.
3. Using a 1.0 mL pipet (#4030), add 0.5 mL R-0890
Molybdenum Buffer Solution to each 25 mL sample
tube. Swirl to mix.
4. Using a separate 1.0 mL pipet (#4029), add 0.5 mL
Molybdenum Indicator Solution (prepared above) to
each sample tube, transferring as few undissolved
crystals as possible. However, a few crystals that
may be transferred will not affect results. Swirl
to mix. The blank should turn peach and the sample
will turn red-orange to red if molybdenum is present.
5. Add R-0892 Molybdenum Titrating Solution, dropwise,
swirling and counting after each drop, to sample
tube containing water sample, until sample color
matches blank color, or until no further change in
color occurs. Always hold bottle in vertical position.
6. Multiply drops of R-0892 Molybdenum Titrating
Solution by 5. Record as parts per million (ppm)
molybdenum.
NOTE: To convert molybdenum (Mo) readings to molybdate
(MoO4), multiply Mo readings by 1.7; to convert
to sodium molybdate dihydrate (Na2MoO4.2H2O),
multiply by 2.52.
For 1 drop = 20 or 50 ppm Mo
1. Rinse and fill a clean 25 mL sample tube (#9198) to
25 mL mark with distilled, deionized, or
molybdenum-free tap water. This will be the blank.
2. Using a 3 mL pipet (#4078), place water to be
tested in a second clean 25 mL sample tube.
NOTE:
For 1 drop = 20 ppm, fill pipet to 2.5 mL mark.
For 1 drop = 50 ppm, fill pipet to 1.0 mL mark.
3. Dilute to 25 mL mark with distilled, deionized, or
molybdenum-free tap water.
4. Using a 1.0 mL pipet (#4030), add 1.0 mL R-0890
Molybdenum Buffer Solution to each 25 mL sample
tube. Swirl to mix.
5. Using a separate 1.0 mL pipet (#4029), add 1.0 mL
Molybdenum Indicator Solution (prepared above) to
each sample tube, transferring as few undissolved
crystals as possible. However, a few crystals
that may be transferred will not affect results.
Swirl to mix. The blank should turn peach and the
sample will turn red-orange to red if molybdenum
is present.
6. Add R-0892 Molybdenum Titrating Solution dropwise,
swirling and counting after each drop, to sample
tube containing water sample, until sample color
matches blank color, or until no further change in
color occurs. Always hold bottle in vertical
position.
7.
For 2.5 mL sample, multiply drops of R-0892
Molybdenum Titrating Solution by 20. Record as
parts per million (ppm) molybdenum.
For 1.0 mL sample, multiply drops R-0892 Molybdenum
Titrating Solution by 50. Record as ppm
molybdenum.
NOTE: To convert molybdenum (Mo) readings to molybdate
(MoO4), multiply Mo readings by 1.7; to convert
to sodium molybdate dihydrate (Na2MoO4.2H2O),
multiply by 2.52.
This product ships within approx. 5 to 7 business days from the state of Maryland. Listed below is the shipping transit time for 2024. UPS transit map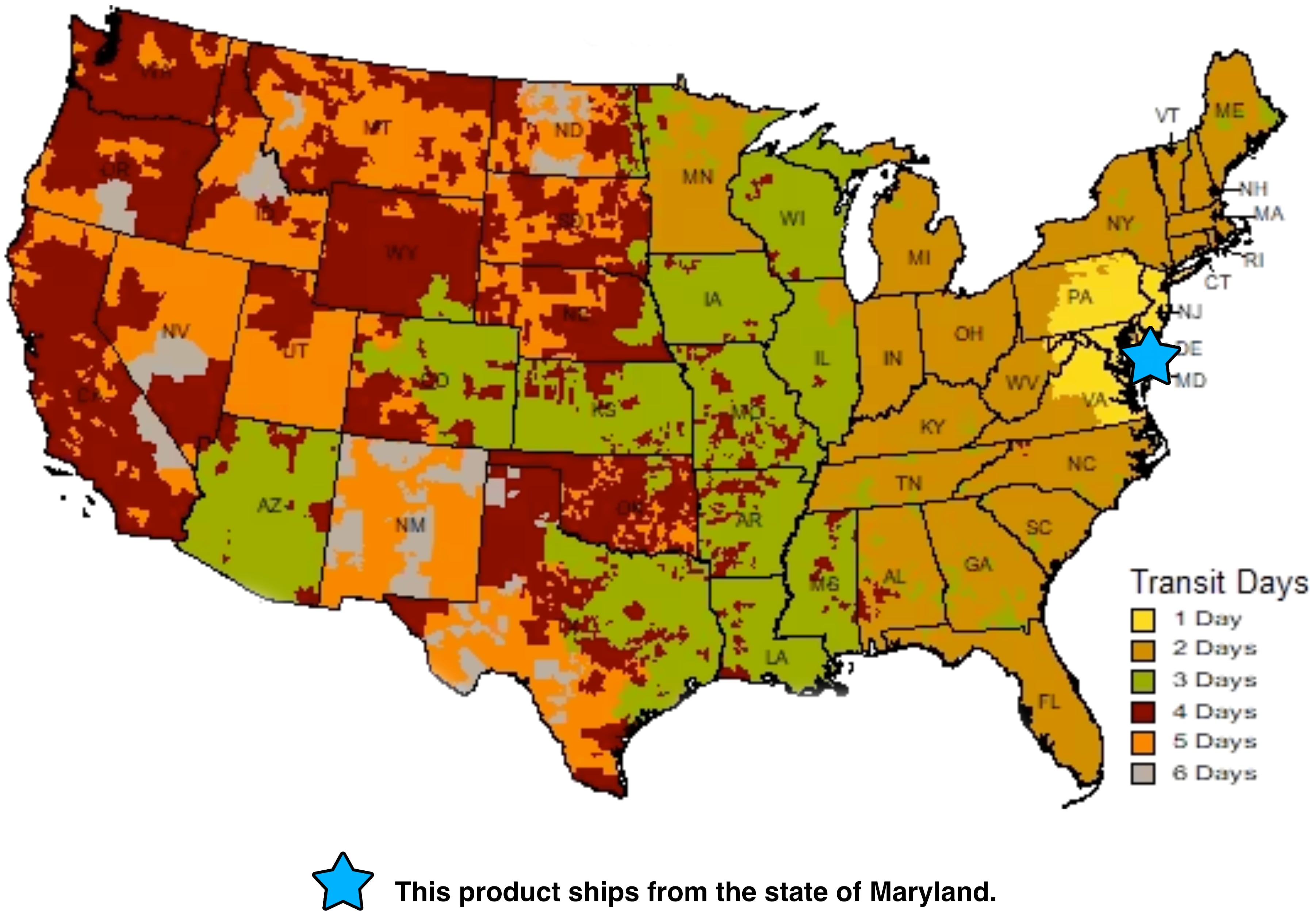 - This product only ships UPS. We can not ship this product via Fedex or USPS.
- We can ship internationally.
- We can not ship this product to a PO box.
- If the product is Haz mat. It can not can not ship this via Air.
You will receive the tracking info after the order ships within 48 to 72 hours. We do not offer expedited shipping for this product.
If you can not order online, call in your order at 800-658-7716.
|
|
 |
 |
 |
 |

|